| Sterile operation | ||
Sterile operation and sterile seals. A great many products could not be manufactured without germfree equipment capable of being sterilized. In addition to having the right technical data, mechanical seals for sealing the drive shafts of such machinery must also display a host of additional properties derived from general requirements on cleanliness, legislative measures and aspects of health policy. In the current absence of German and European regulations, reference is taken for the most part from the FDA (Food and Drug Administration), the USDA (U.S. Department of Agriculture) and the 3-A Sanitary Standards. The latter contain general notes on approved materials and surfaces for the food sector (milk and dairy products). They are often used for want of other standards, but contain little concrete information. Contents deal solely with stainless steels, carbon, ceramic, rubber-elastic and plastic materials, metals and metal oxides, which under conditions of the planned application have to be as corrosion-resistant, non-toxic and non-absorbent as steels of the AISI 300 series. The following materials are used in the main for mechanical seals in sterile operations and can be issued with clearance certificates: Face material pairings Product side:
|
Atmosphere side:
O-rings, secondary seals (only all-elastomer materials approved) Ethylen propylene E867-60 blue, E857-70 green, E883-80 blue. Fluorocarbon V859-70 green. Silicone S797-40 red, S604-70 USDA No. 1800, S229-40 white. Mechanical seal for agitators, driers, fillers etc. in sterile operations, with
rotating counter ring on product side |
Pump mechanical seal for sterile operation: single,
product-lubricated rolled bellows, electropolished finish to all metallic surfaces in
contact with the product, rotating counter ring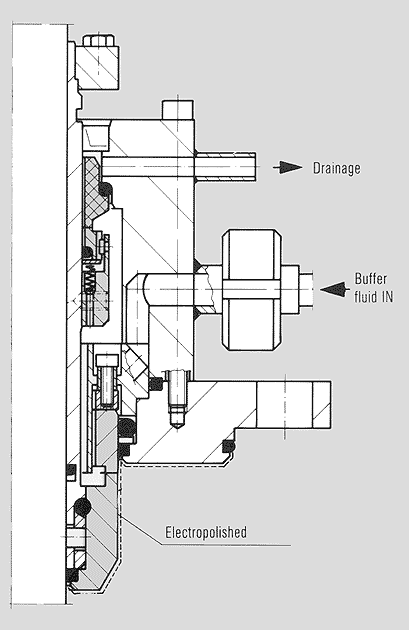 Sealing systems for sterile operation – Sectors, processes and requirements |